Artificial Intelligence (AI) has revolutionized healthcare, particularly in medical image analysis. However, the “black-box” nature of deep learning models remains a significant barrier to their adoption in clinical settings. Clinicians demand not only accuracy but also transparency and interpretability—they need to understand why an AI system makes a particular diagnosis.
In response to this challenge, researchers Yuchen Jiang, Xinyuan Zhao, Yihang Wu, and Ahmad Chaddad from the Artificial Intelligence for Personalised Medicine Lab at Guilin University of Electronic Technology have proposed a novel solution: a Knowledge Distillation-Based Approach to Enhance Transparency of Classifier Models.
This groundbreaking research, detailed in their paper A Knowledge Distillation-Based Approach to Enhance Transparency of Classifier Models , introduces a method that simplifies complex Convolutional Neural Networks (CNNs) while preserving high accuracy and enabling intuitive visual explanations.
Let’s dive into how this Knowledge Distillation + Explainable AI (XAI) hybrid model works, why it matters for healthcare, and what it means for the future of trustworthy medical AI.
Why Transparency Matters in Medical AI
Before we explore the technical details, it’s essential to understand the why behind this research.
In medicine, a misdiagnosis can be life-threatening. When an AI model flags a brain tumor or detects early signs of Alzheimer’s, doctors must trust the result. But if the model cannot explain its reasoning, skepticism arises.
This is where Explainable AI (XAI) comes in. XAI methods like Grad-CAM and SHAP help visualize which parts of an image influenced the model’s decision. But these tools often struggle with large, complex models due to high computational costs and information overload.
Enter Knowledge Distillation (KD)—a technique that transfers knowledge from a large “teacher” model to a smaller, more efficient “student” model.
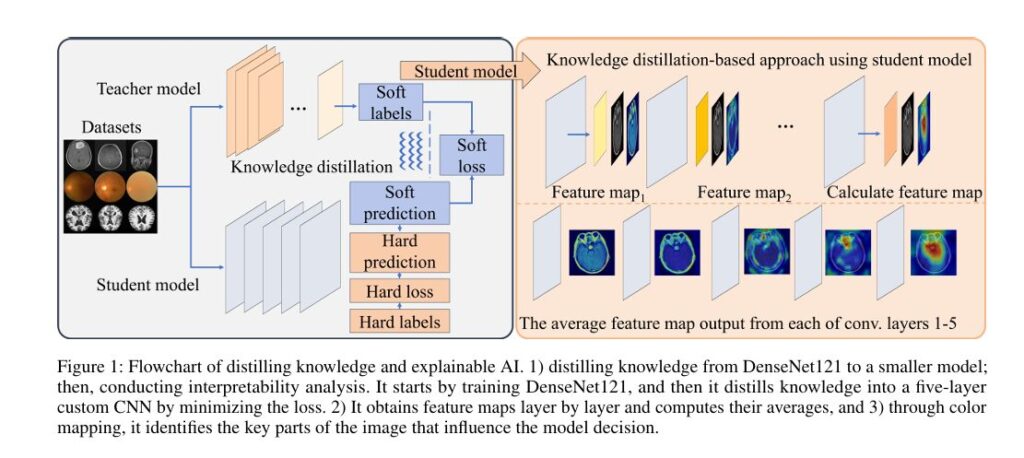
The team’s innovation lies in combining KD with layer-wise feature map visualization, creating a lightweight, accurate, and highly interpretable AI system tailored for medical diagnostics.
The KD-FMV Framework: Simplifying AI Without Sacrificing Performance
The proposed method, called Knowledge Distillation and Feature Map Visualization (KD-FMV), follows a two-step pipeline:
- Train a powerful teacher model (e.g., DenseNet121) on medical image datasets.
- Distill its knowledge into a smaller CNN-based student model using soft and hard loss functions.
- Analyze the student model layer-by-layer using averaged feature maps to reveal decision-making processes.
This approach achieves three critical goals:
- ✅ High classification accuracy
- ✅ Reduced model complexity
- ✅ Enhanced interpretability
Let’s break down each component.
Step 1: Knowledge Distillation – Learning from the Expert
Knowledge Distillation works by training a smaller student model to mimic the behavior of a larger, pre-trained teacher model. The teacher has learned rich feature representations from vast data (e.g., ImageNet), and the student learns not just from labels, but from the teacher’s softened output probabilities.
The total loss function used in KD-FMV is a weighted combination of two components:
Hard Loss (Cross-Entropy with True Labels)
\[ \ell_{HL} = -\frac{1}{N} \sum_{i=1}^{N} \sum_{c=1}^{M} y_{i,c} \, \log \big( y^{S}_{i,c} \big) \]Where:
- N : number of samples
- M : number of classes
- yi , c : true label (one-hot encoded)
- yS,i,c : student model’s predicted probability
Soft Loss (KL Divergence with Teacher Output)
\[ \ell_{\text{SL}}(y^{\text{true}}, y^{T}, T) = – \sum_{c=1}^{M} \; \text{softmax}(T \, y^{\text{true}}_{c}) \, \log \big( \text{softmax}(T \, y^{T}_{c}) \big) \]Here, T is the temperature parameter, controlling the softness of the probability distribution. Higher T values make the distribution smoother, allowing the student to learn nuanced patterns.
Combined Distillation Loss
\[ L_{\text{distill}} = \alpha \cdot \ell_{HL} + (1 – \alpha) \cdot \ell_{SL} \]
Where α balances the influence of ground truth labels and teacher knowledge.
By tuning α and T , the student model achieves performance close to—or even surpassing—the teacher.
Step 2: Interpretable Analysis via Average Feature Maps
One of the biggest challenges in XAI is information overload. Deep CNNs produce dozens of feature maps per layer, making it hard to extract meaningful insights.
KD-FMV solves this by computing the average feature map across all filters in a given layer:
\[ A(i,j) = \sum_{k=1}^{N_1} F_k(i,j) \]Where:
\[ A(i,j) \; : \; \text{average activation at spatial position } (i,j) \] \[ F_k(i,j) \; : \; \text{activation from the $k$-th filter} \] \[ N \; : \; \text{total number of filters in the layer} \]This simple yet effective technique generates a single, clean heatmap per layer, highlighting regions that contribute most to the model’s decision.
Testing on Real Medical Datasets: Brain Tumor, Eye Disease, and Alzheimer’s
To validate their approach, the researchers tested KD-FMV on three public medical datasets:
| DATASET | CLASSES | TOTAL IMAGES | TASK |
|---|---|---|---|
| Brain Tumor MRI | Glioma, Meningioma, No Tumor, Pituitary | 7,023 | Tumor Classification |
| Eye Disease (CFP) | Cataract, Diabetic Retinopathy, Glaucoma, Normal | 4,217 | Fundus Image Diagnosis |
| Alzheimer’s MRI | Mild, Moderate, Non-demented, Very Mild | ~34,000 | Dementia Staging |
Each dataset was split into 70% training, 20% validation, 10% test (except Brain Tumor, which used an 8:2 train-test split).
The teacher model was DenseNet121 pre-trained on ImageNet. The student model was a custom 5-layer CNN with max pooling for feature retention.
Performance Results: Accuracy Meets Efficiency
Despite having far fewer layers, the student models achieved remarkable accuracy, often matching or exceeding the teacher.
Table: Classification Performance Across Datasets
| MODEL | DATASET | ACCURACY | F1-SCORE | LOSS |
|---|---|---|---|---|
| DenseNet121 (Teacher) | Brain Tumor | 0.9877 | 0.99 | 0.0621 |
| Student (Best) | Brain Tumor | 0.9748 | 0.98 | 0.0944 |
| DenseNet121 (Teacher) | Eye Disease | 0.9837 | 0.99 | 0.0490 |
| Student (Best) | Eye Disease | 0.9351 | 0.94 | 0.1956 |
| DenseNet121 (Teacher) | Alzheimer | 0.9938 | 0.99 | 0.0247 |
| Student (Best) | Alzheimer | 0.9946 | 0.99 | 0.0194 |
Notably, in the Alzheimer’s dataset, the student model outperformed the teacher—likely due to optimal temperature tuning during distillation.
Interpretability Analysis: Seeing How the Model Thinks
To evaluate interpretability, the team used two established XAI methods:
- Grad-CAM: Highlights regions in the final convolutional layer that most influence the prediction.
- SHAP (SHapley Additive exPlanations): Quantifies the contribution of each pixel to the final output.
They compared these with their proposed method (KD-FMV) using Fidelity Score, which measures how well an explanation aligns with the original model’s behavior.
Table: Fidelity Scores by Method and Dataset
| DATASET | CLASS | GRAD-CAM | SHAP | OURS (KD-FMV) |
|---|---|---|---|---|
| Brain Tumor | Glioma | 0.9639 | 0.9524 | 0.9764 |
| Meningioma | 0.8934 | 0.9687 | 0.9161 | |
| Pituitary | 0.9322 | 0.9002 | 0.9161 | |
| Average | 0.9298 | 0.9404 | 0.9277 | |
| Eye Disease | Cataract | 0.8555 | 0.7733 | 0.7852 |
| Diabetic Retinopathy | 0.9684 | 0.9556 | 0.9080 | |
| Glaucoma | 0.8939 | 0.8814 | 0.8958 | |
| Average | 0.9059 | 0.8700 | 0.8630 | |
| Alzheimer | Mild Demented | 0.9905 | 0.9815 | 0.9613 |
| Moderate Demented | 0.9898 | 0.9666 | 0.9508 | |
| Very Mild Demented | 0.7533 | 0.8595 | 0.8274 | |
| Average | 0.9112 | 0.9359 | 0.9132 |
While SHAP showed slightly higher fidelity in some cases, KD-FMV delivered competitive results with significantly lower computational cost.
Time-Efficient Interpretability: Faster Insights for Faster Care
One of the most compelling advantages of KD-FMV is its computational efficiency.
Using Floating Point Operations (FLOPs) and Mean Execution Time (MET), the team showed that the student model requires less than 50% of the resources of the teacher.
Table: Computational Efficiency Comparison
| MODEL | DATASET | FLOPS (x 106) | MET-GRAD-CAM (S) | MET-SHAP (S) |
|---|---|---|---|---|
| Teacher | Brain Tumor | 566.89 | 0.9521 | 67.68 |
| Student | Brain Tumor | 232.70 | 0.1963 | 15.64 |
| Teacher | Eye Disease | 566.89 | 0.9389 | 67.56 |
| Student | Eye Disease | 232.70 | 0.1899 | 15.40 |
| Teacher | Alzheimer | 566.89 | 0.9346 | 68.46 |
| Student | Alzheimer | 232.70 | 0.1941 | 15.63 |
💡 Key Insight: SHAP analysis dropped from ~68 seconds to ~15 seconds per image—a 78% reduction in time. This is crucial for hospitals processing thousands of scans daily.
Why This Matters: The Future of Trustworthy Medical AI
The KD-FMV framework addresses three major challenges in deploying AI in healthcare:
- Model Complexity → Reduced via Knowledge Distillation
- Lack of Transparency → Solved with layer-wise feature visualization
- High Computational Cost → Minimized through lightweight architecture
Clinicians can now:
- View heatmaps showing tumor locations in MRI scans
- Track how features evolve across layers (edges → textures → objects)
- Make faster, more informed decisions based on explainable outputs
Moreover, the student model’s small size makes it ideal for deployment on mobile devices, edge computing systems, or telemedicine platforms—bringing AI-powered diagnostics to remote areas.
Call to Action: Explore the Code and Join the Movement
This research isn’t just theoretical—it’s open-source and ready for real-world use.
👉 Access the full code on GitHub: https://github.com/AIPMLab/KD-FMV
You can:
- Replicate the experiments
- Apply KD-FMV to your own medical datasets
- Contribute to the development of transparent AI in healthcare
Whether you’re a researcher, clinician, or developer, this project offers a blueprint for building accurate, efficient, and trustworthy AI models.
Conclusion: Bridging the Gap Between AI and Clinical Trust
The integration of Knowledge Distillation and Explainable AI in the KD-FMV framework represents a major step forward in medical AI. By simplifying complex models without sacrificing performance, and by enabling intuitive, layer-by-layer interpretation, this approach enhances both accuracy and trust.
As AI continues to transform medicine, transparency must be non-negotiable. Methods like KD-FMV ensure that AI doesn’t just predict—it explains, helping doctors make better decisions and ultimately improving patient outcomes.
Let’s move beyond black-box models. Let’s build AI that doctors can understand, trust, and use—every single day.
I will provide you with the complete end-to-end Python code for the model proposed in the paper “A Knowledge Distillation-Based Approach to Enhance Transparency of Classifier Models.
import tensorflow as tf
from tensorflow.keras.layers import Conv2D, MaxPooling2D, Flatten, Dense, Input, GlobalAveragePooling2D
from tensorflow.keras.models import Model
from tensorflow.keras.applications import DenseNet121
from tensorflow.keras.losses import CategoricalCrossentropy, KLDivergence
from tensorflow.keras.optimizers import Adam
import numpy as np
import matplotlib.pyplot as plt
class KD_FMV:
"""
Implements the Knowledge Distillation and Feature Map Visualization (KD-FMV)
approach described in the research paper.
"""
def __init__(self, input_shape=(224, 224, 3), num_classes=4, temperature=10, alpha=0.7):
"""
Initializes the teacher and student models, and the distiller.
Args:
input_shape (tuple): The shape of the input images.
num_classes (int): The number of classes for classification.
temperature (int): The temperature for softening probabilities.
alpha (float): The weight to balance hard and soft loss.
"""
self.input_shape = input_shape
self.num_classes = num_classes
self.temperature = temperature
self.alpha = alpha
# 1. Define Teacher Model (DenseNet121)
self.teacher_model = self._build_teacher_model()
# 2. Define Student Model (Simplified CNN)
self.student_model = self._build_student_model()
# 3. Combine into a Distiller model
self.distiller = Distiller(student=self.student_model, teacher=self.teacher_model, temperature=self.temperature, alpha=self.alpha)
def _build_teacher_model(self):
"""
Builds the teacher model using a pre-trained DenseNet121.
"""
base_teacher = DenseNet121(weights='imagenet', include_top=False, input_shape=self.input_shape)
base_teacher.trainable = False # Start with pre-trained weights frozen
inputs = Input(shape=self.input_shape)
x = base_teacher(inputs, training=False)
x = GlobalAveragePooling2D()(x)
outputs = Dense(self.num_classes, activation='softmax')(x)
teacher_model = Model(inputs, outputs, name="teacher_model")
print("--- Teacher Model Summary ---")
teacher_model.summary()
return teacher_model
def _build_student_model(self):
"""
Builds the 5-layer student CNN model as described in the paper.
"""
inputs = Input(shape=self.input_shape, name="student_input")
# Layer 1
x = Conv2D(32, (3, 3), activation='relu', padding='same', name='conv1')(inputs)
x = MaxPooling2D((2, 2))(x)
# Layer 2
x = Conv2D(64, (3, 3), activation='relu', padding='same', name='conv2')(x)
x = MaxPooling2D((2, 2))(x)
# Layer 3
x = Conv2D(128, (3, 3), activation='relu', padding='same', name='conv3')(x)
x = MaxPooling2D((2, 2))(x)
# Layer 4
x = Conv2D(256, (3, 3), activation='relu', padding='same', name='conv4')(x)
x = MaxPooling2D((2, 2))(x)
# Layer 5
x = Conv2D(512, (3, 3), activation='relu', padding='same', name='conv5')(x)
x = MaxPooling2D((2, 2))(x)
# Flatten and Dense layers
x = Flatten()(x)
outputs = Dense(self.num_classes, name="student_output")(x) # No softmax here, as it's handled in the loss
student_model = Model(inputs, outputs, name="student_model")
print("\n--- Student Model Summary ---")
student_model.summary()
return student_model
def compile_and_train(self, train_data, epochs=10, learning_rate=1e-4):
"""
Compiles and trains the distiller model.
Args:
train_data (tf.data.Dataset): The training dataset.
epochs (int): Number of epochs to train for.
learning_rate (float): Learning rate for the optimizer.
"""
self.distiller.compile(
optimizer=Adam(learning_rate=learning_rate),
metrics=['accuracy'],
student_loss_fn=CategoricalCrossentropy(from_logits=True),
distillation_loss_fn=KLDivergence(),
)
print("\n--- Starting Student Model Training (Knowledge Distillation) ---")
self.distiller.fit(train_data, epochs=epochs)
print("--- Student Model Training Complete ---")
def visualize_feature_maps(self, image):
"""
Visualizes the average feature map for each convolutional layer in the student model.
Args:
image (np.array): A single input image of shape (1, H, W, C).
"""
print(f"\n--- Generating Feature Map Visualizations for a sample image ---")
if image.ndim == 3:
image = np.expand_dims(image, axis=0)
if image.shape != (1,) + self.input_shape:
raise ValueError(f"Input image must have shape (1, {self.input_shape[0]}, {self.input_shape[1]}, {self.input_shape[2]}) but got {image.shape}")
# Names of the convolutional layers in the student model
layer_names = [layer.name for layer in self.student_model.layers if 'conv' in layer.name]
# Create a model that will return the feature maps
feature_map_model = Model(inputs=self.student_model.inputs, outputs=[self.student_model.get_layer(name).output for name in layer_names])
# Get the feature maps for the input image
feature_maps = feature_map_model.predict(image)
# Plot the original image and the average feature maps
num_layers = len(layer_names)
plt.figure(figsize=(15, 4))
# Original Image
plt.subplot(1, num_layers + 1, 1)
plt.title('Original Image')
plt.imshow(image[0].astype('uint8'))
plt.axis('off')
# Average Feature Maps
for i, (layer_name, fmap) in enumerate(zip(layer_names, feature_maps)):
# Calculate the average feature map across all filters
avg_fmap = np.mean(fmap[0], axis=-1)
plt.subplot(1, num_layers + 1, i + 2)
plt.title(f'Avg FMap: {layer_name}')
plt.imshow(avg_fmap, cmap='viridis')
plt.axis('off')
plt.tight_layout()
plt.show()
class Distiller(Model):
"""
The Distiller model that combines the teacher and student for training.
"""
def __init__(self, student, teacher, temperature, alpha):
super(Distiller, self).__init__()
self.student = student
self.teacher = teacher
self.temperature = temperature
self.alpha = alpha
def compile(self, optimizer, metrics, student_loss_fn, distillation_loss_fn):
super(Distiller, self).compile(optimizer=optimizer, metrics=metrics)
self.student_loss_fn = student_loss_fn
self.distillation_loss_fn = distillation_loss_fn
def train_step(self, data):
x, y = data
# Get teacher predictions (soft labels)
teacher_predictions = self.teacher(x, training=False)
with tf.GradientTape() as tape:
# Get student predictions
student_predictions = self.student(x, training=True)
# Calculate the two losses
student_loss = self.student_loss_fn(y, student_predictions)
distillation_loss = self.distillation_loss_fn(
tf.nn.softmax(teacher_predictions / self.temperature, axis=1),
tf.nn.softmax(student_predictions / self.temperature, axis=1),
)
# Combine the losses
total_loss = self.alpha * student_loss + (1 - self.alpha) * distillation_loss
# Compute gradients
trainable_vars = self.student.trainable_variables
gradients = tape.gradient(total_loss, trainable_vars)
# Update weights
self.optimizer.apply_gradients(zip(gradients, trainable_vars))
# Update metrics
self.compiled_metrics.update_state(y, tf.nn.softmax(student_predictions, axis=1))
# Return a dict of performance
results = {m.name: m.result() for m in self.metrics}
results.update(
{"student_loss": student_loss, "distillation_loss": distillation_loss, "total_loss": total_loss}
)
return results
def test_step(self, data):
x, y = data
y_prediction = self.student(x, training=False)
student_loss = self.student_loss_fn(y, y_prediction)
self.compiled_metrics.update_state(y, tf.nn.softmax(y_prediction, axis=1))
results = {m.name: m.result() for m in self.metrics}
results.update({"student_loss": student_loss})
return results
# --- Main Execution ---
if __name__ == '__main__':
# --- 1. Configuration ---
IMG_HEIGHT = 224
IMG_WIDTH = 224
CHANNELS = 3
NUM_CLASSES = 4 # Example: Brain Tumor (glioma, meningioma, no tumor, pituitary)
BATCH_SIZE = 16
EPOCHS = 5 # Using a small number for a quick demo
TEMPERATURE = 15
ALPHA = 0.4
# --- 2. Create a Dummy Dataset (replace with your actual data loading) ---
print("--- Creating a dummy dataset for demonstration ---")
# Generate random images and one-hot encoded labels
num_samples = 100
dummy_images = np.random.randint(0, 256, size=(num_samples, IMG_HEIGHT, IMG_WIDTH, CHANNELS), dtype=np.uint8)
dummy_labels_indices = np.random.randint(0, NUM_CLASSES, size=(num_samples,))
dummy_labels_one_hot = tf.keras.utils.to_categorical(dummy_labels_indices, num_classes=NUM_CLASSES)
# Create a tf.data.Dataset
train_dataset = tf.data.Dataset.from_tensor_slices((dummy_images, dummy_labels_one_hot))
train_dataset = train_dataset.shuffle(buffer_size=num_samples).batch(BATCH_SIZE)
print(f"Dataset created with {num_samples} samples.")
# --- 3. Initialize and Train the Model ---
kd_model = KD_FMV(
input_shape=(IMG_HEIGHT, IMG_WIDTH, CHANNELS),
num_classes=NUM_CLASSES,
temperature=TEMPERATURE,
alpha=ALPHA
)
# Train the student model using knowledge distillation
kd_model.compile_and_train(train_dataset, epochs=EPOCHS)
# --- 4. Visualize Feature Maps for a Sample Image ---
# Get one batch from the dataset to use for visualization
for images, labels in train_dataset.take(1):
sample_image = images[0]
kd_model.visualize_feature_maps(sample_image.numpy())
break
Related posts, You May like to read
- 7 Shocking Truths About Knowledge Distillation: The Good, The Bad, and The Breakthrough (SAKD)
- 7 Revolutionary Breakthroughs in Medical Image Translation (And 1 Fatal Flaw That Could Derail Your AI Model)
- DeepSPV: Revolutionizing 3D Spleen Volume Estimation from 2D Ultrasound with AI
- ACAM-KD: Adaptive and Cooperative Attention Masking for Knowledge Distillation
- GeoSAM2 3D Part Segmentation — Prompt-Controllable, Geometry-Aware Masks for Precision 3D Editing
- Enhancing Vision-Audio Capability in Omnimodal LLMs with Self-KD

Pingback: MedDINOv3: Revolutionizing Medical Image Segmentation with Adaptable Vision Foundation Models - aitrendblend.com